Eight new substances candidates for Annex XIV
ECHA invites comments on its proposal to include eight substances of very high concern in the list of REACH authorisations.
ECHA invites to send by 2 May 2022 the observations on eight substances of very high concern candidates to enter Annex XIV of the REACH Regulation:
- Ethylenediamine (CAS 107-15-3) used as a corrosion inhibitor, processing aid, used in odour emission control
- Lead (CAS 7439-92-1) used in batteries' manufacture, solders, certain metal alloys (e.g., brass), in the manufacture of automobile parts and electronic devices.
- Glutaraldehyde (CAS 111-30-8) used as a detergent and biocide, agent for tanning leather, in polymers and in the development of X-ray films
- 2-(4-tertbutylbenzyl)propionaldehyde used as a fragrance in cleaning products and environmental perfumers, and in cosmetics
- Diisohexylphthalate (CAS 71850-09-4 ) used as lubricant and plasticizer
- Orthoboric acid sodium salt (CAS 13840-56-7) used as solvent and corrosion inhibitor
- 2-methyl-1-(4-methylthiophenyl)-2-morpholinopropan-1-one (CAS 71868-10-5) used as photo-initiator in UV curing paints, inks and adhesives
- 2-Benzyl-2-dimethylamino-4'-morpholinobutyrophenone used as photo-initiator in UV curing paints, inks and adhesives
The authorisation process consists of four phases:
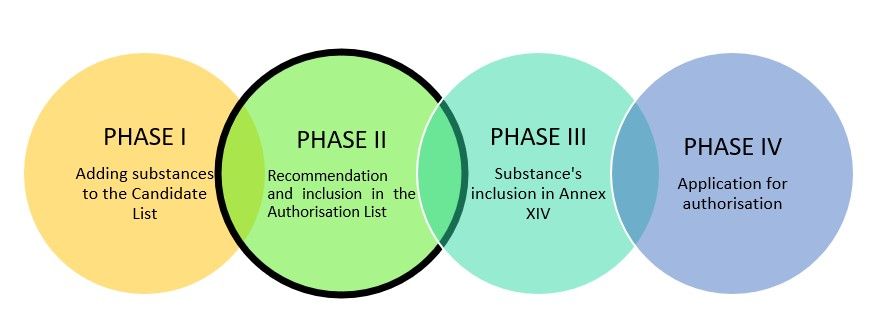
According to Article 58, ECHA invites all interested parties to submit comments within three months of that publication (2 May 2022). These comments shall concern exempted categories from the authorisation requirement; then it should be sent to the Commission an up-to-date recommendation based on the information obtained. Once a substance is added to Annex XIV, to use it a company must apply for authorisation.
After that, the application shall be evaluated by Risk Assessment Committee (RAC) and by Socio-economic Analysis Committee (SEAC), both supported by ECHA Secretariat.
As part of this process, ECHA organises a consultation to gather information on possible alternative substances or technology for the required uses. After approximately 1 year, the application may be accepted if the reasons submitted are valid or rejected as there is evidence of possible alternatives for the substance.
Source: ECHA
The most read news
-
Proposal for "Short-form warning" amendment in the field of Proposition 65
-
Obligation to notify hazardous mixtures also for GB-CLP
-
New Regulation (EU) 477/2022: important changes and new information requirements for chemicals registration
-
Proposal for "Short-form warning" amendment in the field of Proposition 65
-
Announcement for a new European regulation on Sustainable Eco-design
-
Circular Economy: new plans for the textile industries